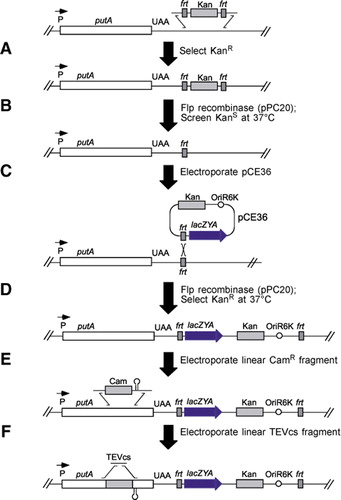
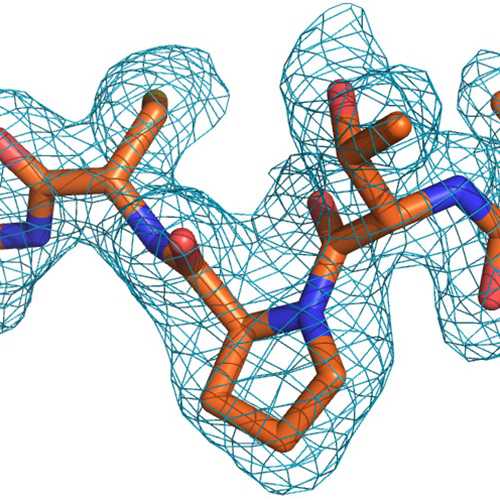
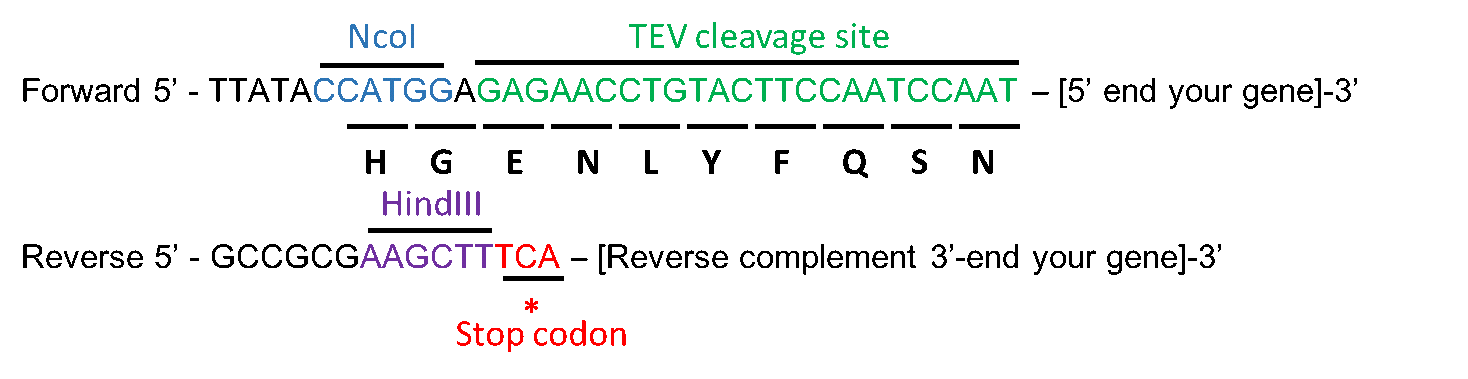
A fully automated procedure for the parallel, multidimensional purification and nucleotide loading of the human GTPases KRas, Rac1 and RalB. - Abstract - Europe PMC
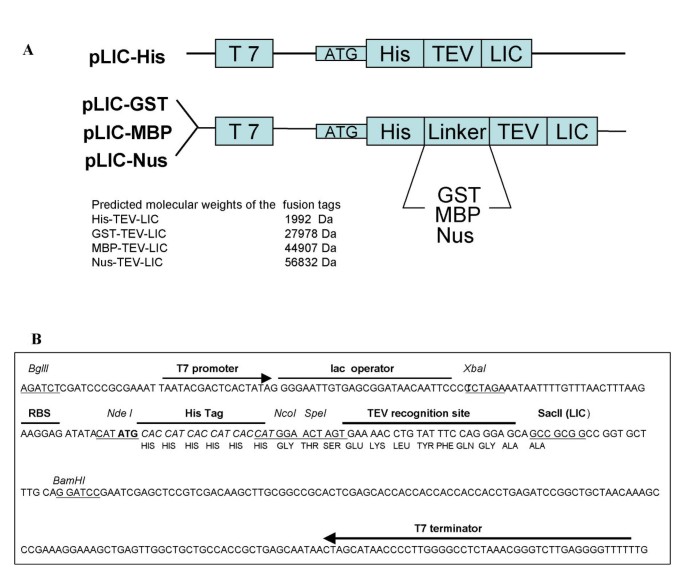
A family of E. coliexpression vectors for laboratory scale and high throughput soluble protein production | BMC Biotechnology | Full Text

YESS 2.0, a Tunable Platform for Enzyme Evolution, Yields Highly Active TEV Protease Variants | ACS Synthetic Biology

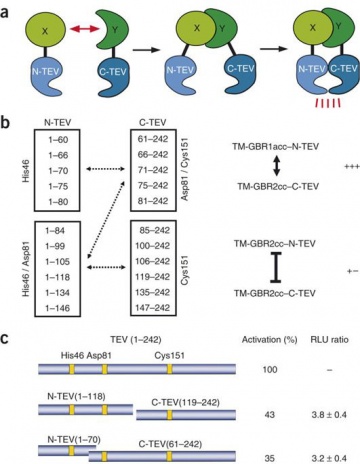
A split protease-E. coli ClpXP system quantifies protein–protein interactions in Escherichia coli cells | Communications Biology
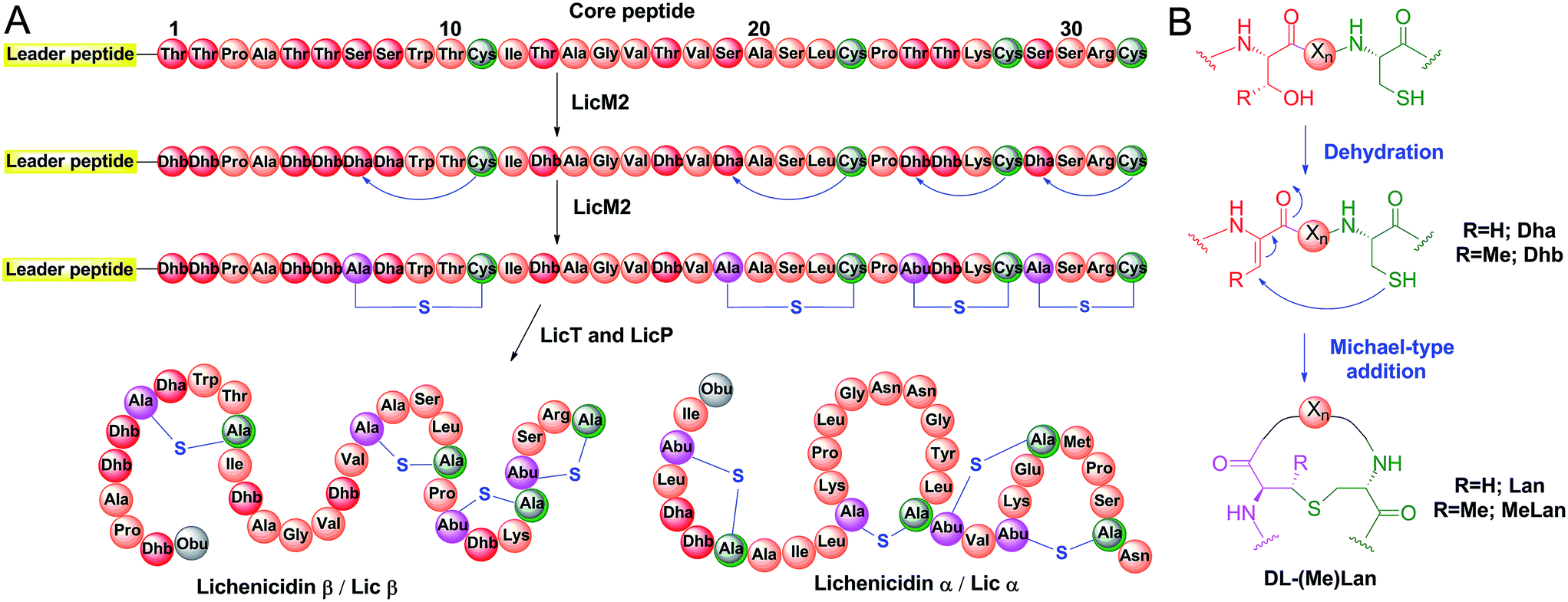
Applications of the class II lanthipeptide protease LicP for sequence-specific, traceless peptide bond cleavage - Chemical Science (RSC Publishing) DOI:10.1039/C5SC02329G
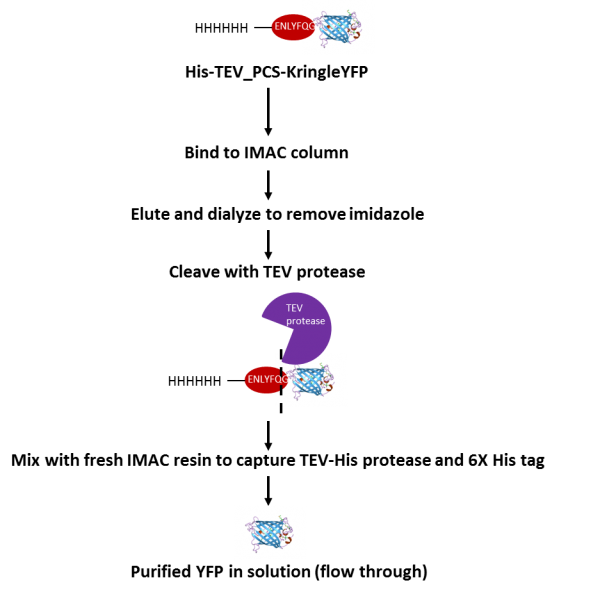
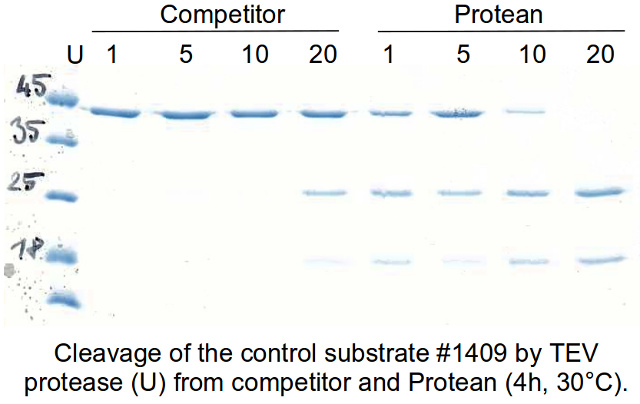
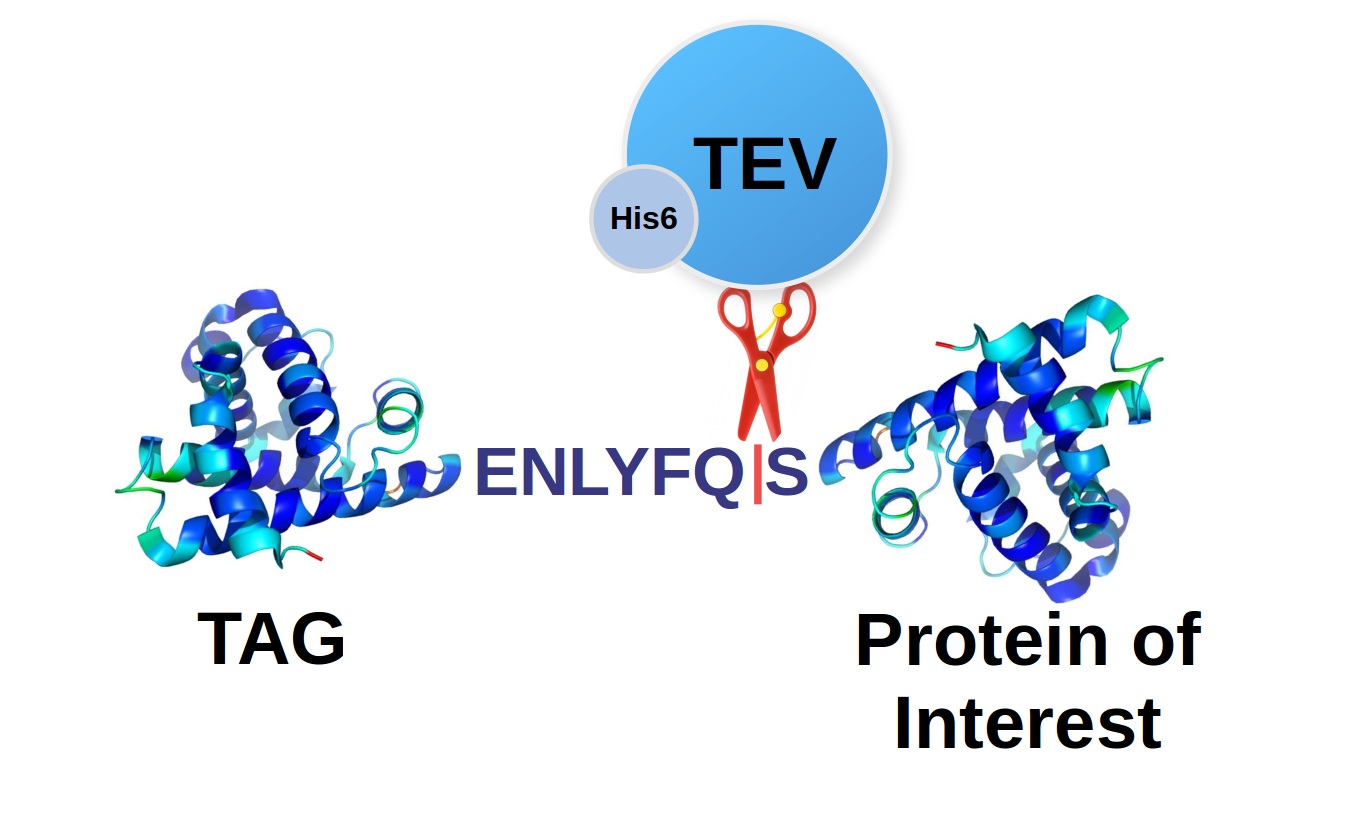
Enzymes - Tobacco Etch Virus (TEV) and Human RhinoVirus (HRV3C) Cysteine Proteases in Vectors | ATUM - ATUM

Phosphorylation regulates proteolytic efficiency of TEV protease detected by a 5(6)-carboxyfluorescein-pyrene based fluorescent sensor - ScienceDirect

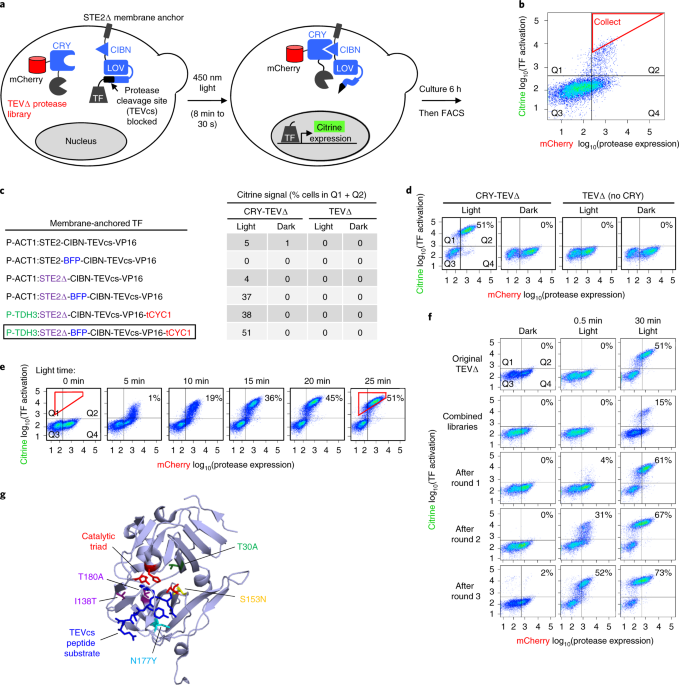
Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries | PNAS