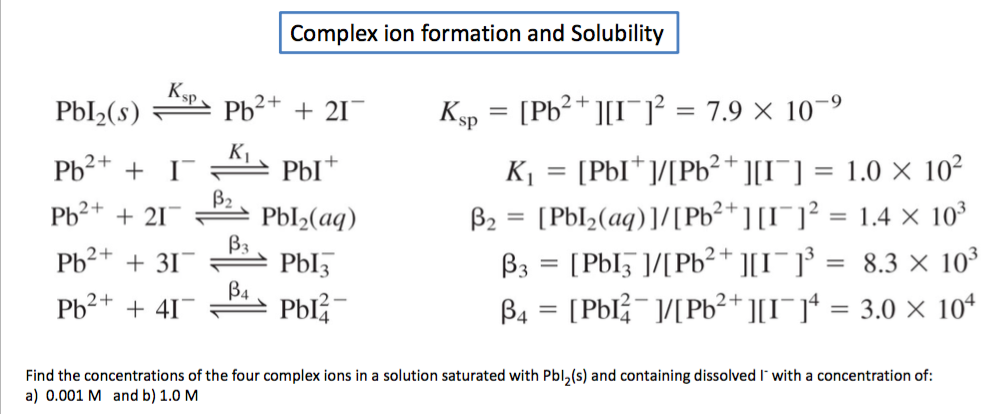
![SOLVED: Given that the solubility product of PbI2 is expressed as Ksp = [Pb2 +][I–]2, calculate the Ksp of PbI2 from the concentration of Pb2+ found in Step 6 of the Data Analysis ( SOLVED: Given that the solubility product of PbI2 is expressed as Ksp = [Pb2 +][I–]2, calculate the Ksp of PbI2 from the concentration of Pb2+ found in Step 6 of the Data Analysis (](https://cdn.numerade.com/ask_previews/e757804a-9046-476b-bdfd-a9c26ea91520_large.jpg)
SOLVED: Given that the solubility product of PbI2 is expressed as Ksp = [Pb2 +][I–]2, calculate the Ksp of PbI2 from the concentration of Pb2+ found in Step 6 of the Data Analysis (
Efficient and selective removal of Pb2+ from aqueous solution by using an O− functionalized metal–organic framework - Dalton Transactions (RSC Publishing)

Efficient Removal of Pb2+ from Aqueous Solution by an Ionic Covalent–Organic Framework: Molecular Simulation Study | Industrial & Engineering Chemistry Research

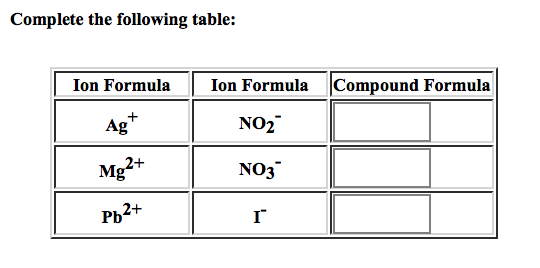
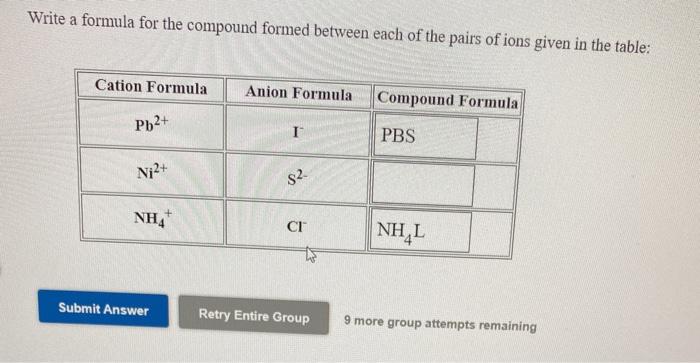
SOLVED: Write the chemical formula and name for each combination of the ions Pb2+ and N-3 > formula and name b Fe3+ and OH" > formula and name C A/3+ and CO3-2 >
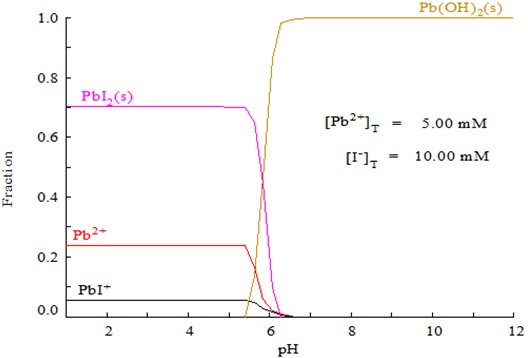
![SOLVED: What [Pb2+] should be maintained in Pb(NO3)(aq) to produce a solubility of 1.9×10−4 mol PbI2/L when PbI2(s) is added? Express your answer using two significant figures. The answers I found in SOLVED: What [Pb2+] should be maintained in Pb(NO3)(aq) to produce a solubility of 1.9×10−4 mol PbI2/L when PbI2(s) is added? Express your answer using two significant figures. The answers I found in](https://cdn.numerade.com/ask_previews/08fd4d3d-df52-4bbe-9540-0ab919346f25.gif)
SOLVED: What [Pb2+] should be maintained in Pb(NO3)(aq) to produce a solubility of 1.9×10−4 mol PbI2/L when PbI2(s) is added? Express your answer using two significant figures. The answers I found in

Pb2+ as a Substrate and a Cofactor of a Porphyrin Metalation DNAzyme - Yang - 2020 - ChemBioChem - Wiley Online Library

SOLVED: 12) Considering the following precipitation reaction: Pb(NO3)2(aq) + 2KI(aq) Pbl2(s) + 2KNO3aq) Which ion would NOT be present in the complete ionic equation? A) I- B) K+ D) Pb2+ C)NO3 E)
![Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: an allosteric effect in extraction - RSC Advances (RSC Publishing) Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: an allosteric effect in extraction - RSC Advances (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C3RA44289F)
Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: an allosteric effect in extraction - RSC Advances (RSC Publishing)




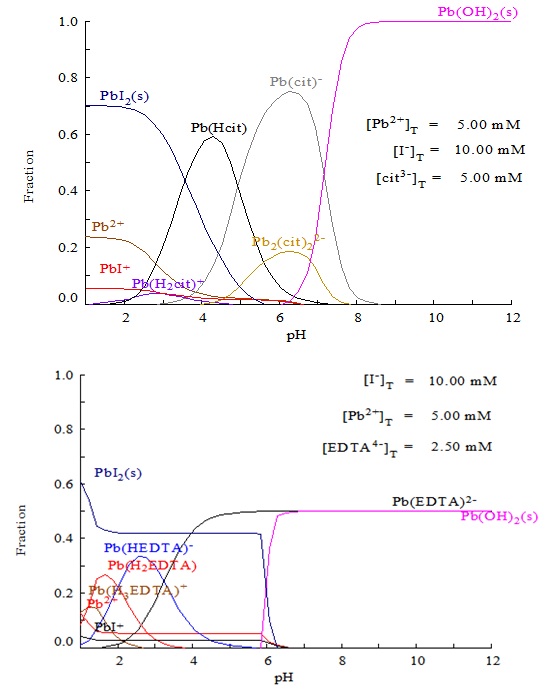





![H2ampa─Versatile Chelator for [203Pb]Pb2+, [213Bi]Bi3+, and [225Ac]Ac3+ | Inorganic Chemistry H2ampa─Versatile Chelator for [203Pb]Pb2+, [213Bi]Bi3+, and [225Ac]Ac3+ | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.2c00636/asset/images/acs.inorgchem.2c00636.social.jpeg_v03)