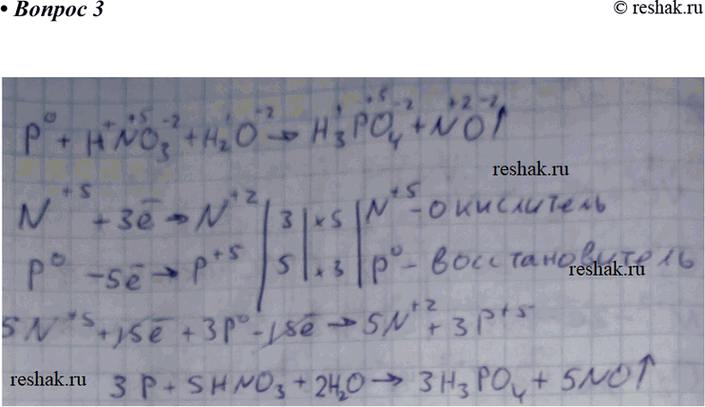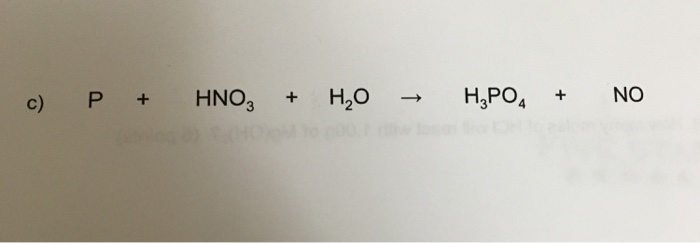When aniline reacts with nitric acid in the presence of acetic anhydride, it gives p-nitroaniline only. Why? - Quora

Algebraic method. P4+HNO3=H3PO4+NO2+H2O balance the equation by a,b,c method. p4+hno3=h3po4+no2+h2o - YouTube

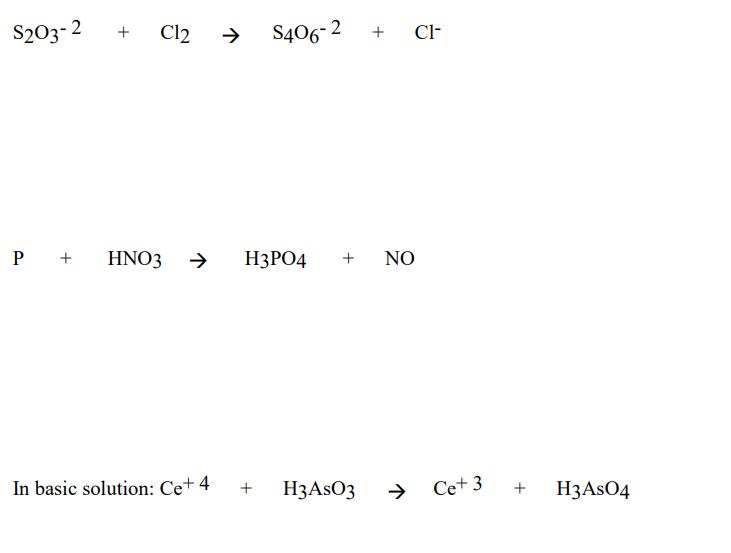
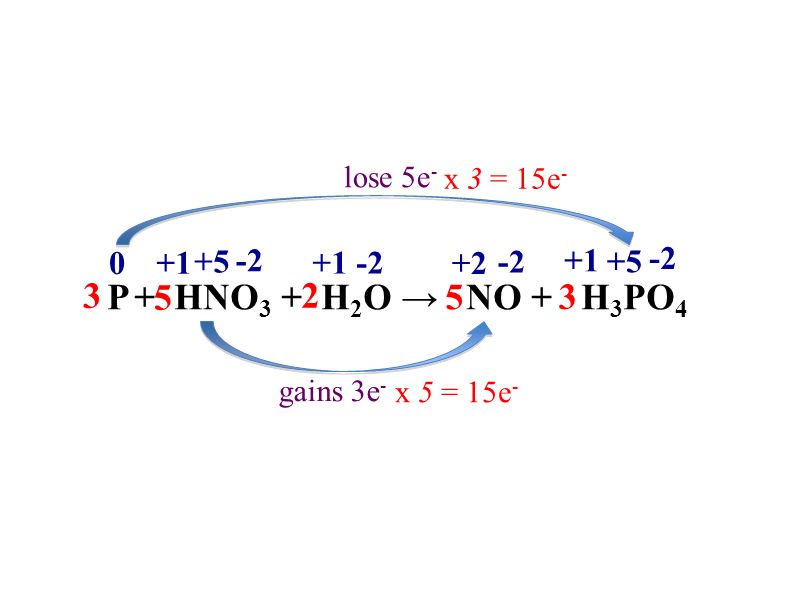
Concentrated nitric acid oxidised phosphorus to phosphoric acid according to the following equation: P+5HNO3(conc.)---> H3PO4 +H2O +5NO2 If 9.3 g of phosphorus was used in the reaction, calculate: I) Number of moles

Используя метод электронного баланса, расставьте коэффициенты в уравнении реакции, схема которой P - Школьные Знания.com

What would be the stoichiometric coefficient of P and HNO 3 respectively, in the net balanced equation for the given redox reaction:P + HNO 3→ HPO 3+ NO + H 2 OA. 2,3B. 5,3C. 1,1D. 3,5

Oxidation Number method. P4+HNO3+H2O=H3PO4+NO. Balance the equation by oxidation Number method. - YouTube

HNO3/H3PO4–NANO2 mediated oxidation of cellulose — preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation - ScienceDirect

Balance the following equation by partial equation method: P4 + HNO3 = H3PO4 + NO2 + H2O | Homework.Study.com

Which of the following on oxidation with alkaline KMnO4 followed by acidification with dil. HCl gives terephthalic acid?