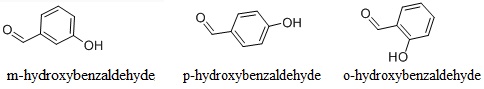
Molecular structures of four o-hydroxybenzaldehyde systems as obtained... | Download Scientific Diagram

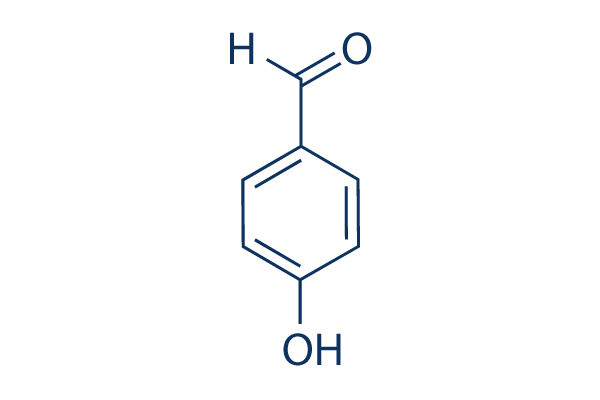
Selective Synthesis of p-Hydroxybenzaldehyde by Liquid-Phase Catalytic Oxidation of p-Cresol | Organic Process Research & Development

1649426-95-8 | Salicylaldehyde-13C6 | Salicylal-13C6; 2-Formylphenol-13C6; 2-Hdroxybenzaldehyde-13C6; 2-Hydroxybenzaldehyde-13C6; NSC 112278-13C6; NSC 49178-13C6; NSC 83559-13C6; NSC 83560-13C6; NSC 83561-13C6; NSC 83562-13C6; NSC 97202-13C6; Salicylal ...

When 0 hydroxybenzaldehyde is heated with ethanoic an hydride in presence sodium ethanoate, compound formed during the reaction is ?

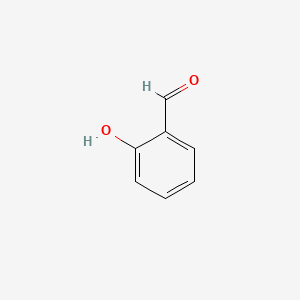
Structural and orthoselectivity study of 2-hydroxybenzaldehyde using spectroscopic analysis - ScienceDirect

o-Hydroxy benzaldehyde is more soluble in water than p-hybroxy benzaldehde (b) o-Hydroxy b - YouTube
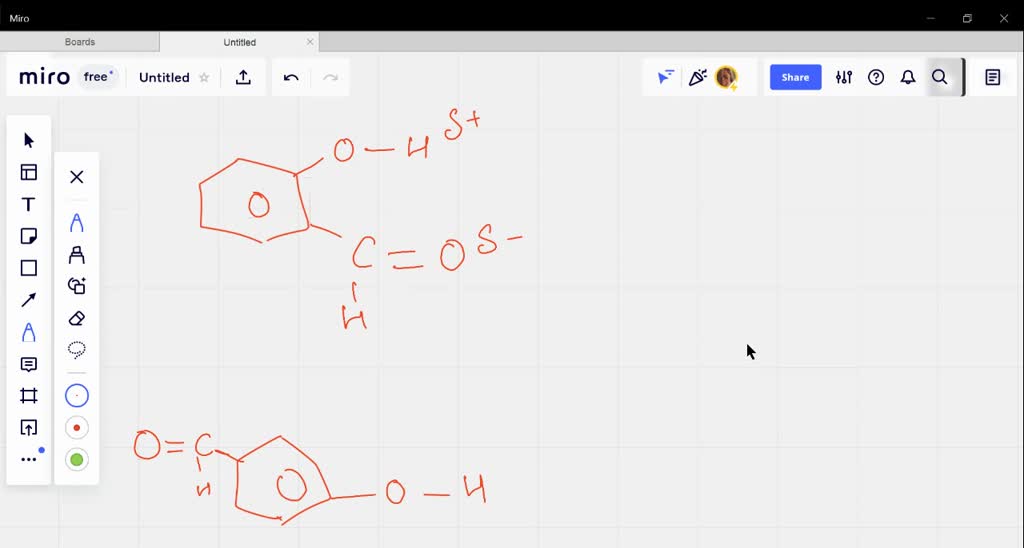
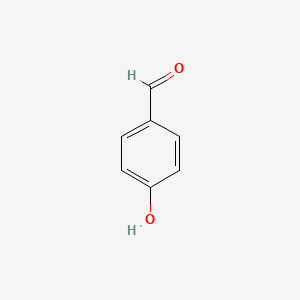
SOLVED:Explain why o-hydroxybenzaldehyde is a liquid at room temperature while p-hydroxybenzaldehyde is a solid?

Explain why o-hydroxybenzaldehyde is a liquid at room temp , while p- hydroxybenzaldehyde is a high melting solid - Chemistry - Chemical Bonding and Molecular Structure - 10498271 | Meritnation.com

o - Hydroxybenzaldehyde is a liquid at room temperature while p - hydroxybenzaldehyde is a high melting solid because of:
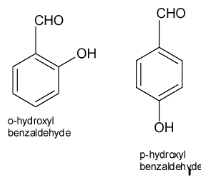
Assertion: Both o-hydroxybenzaldehyde and p-hydroxy benzaldehyde have the same molecular weight and show H-bonding.Reason: Melting point of p- hydroxybenzaldehyde is more than o-hydroxybenzaldehyde.A. Both assertion and reason are correct and reason is the

When benzene in presence of anhydrous aluminium chloride reacts with ethyl chloride the compound formed is:
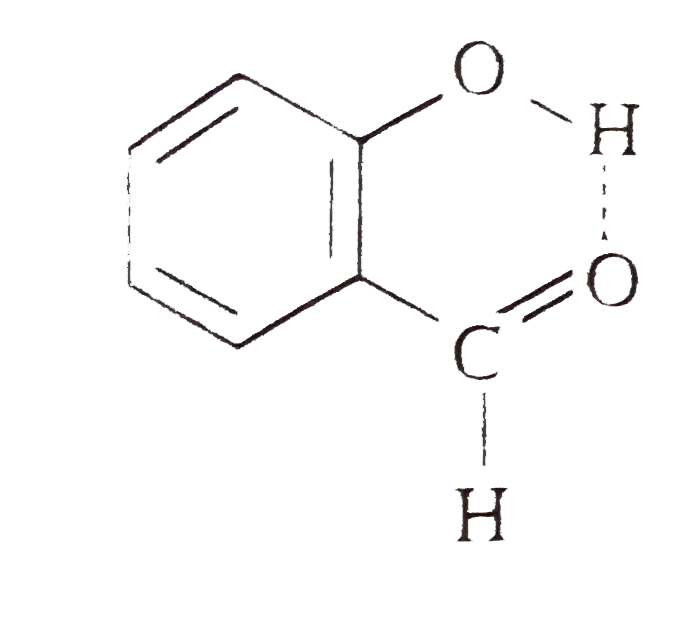
Explain why is o-hydroxybenzaldehyde a liquis at room temperaturre while p- hydroxybenzaldehyde is a high melting solid.