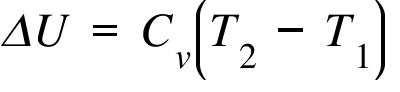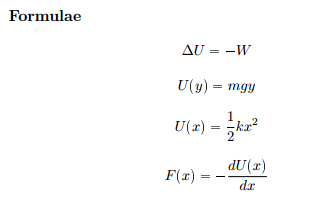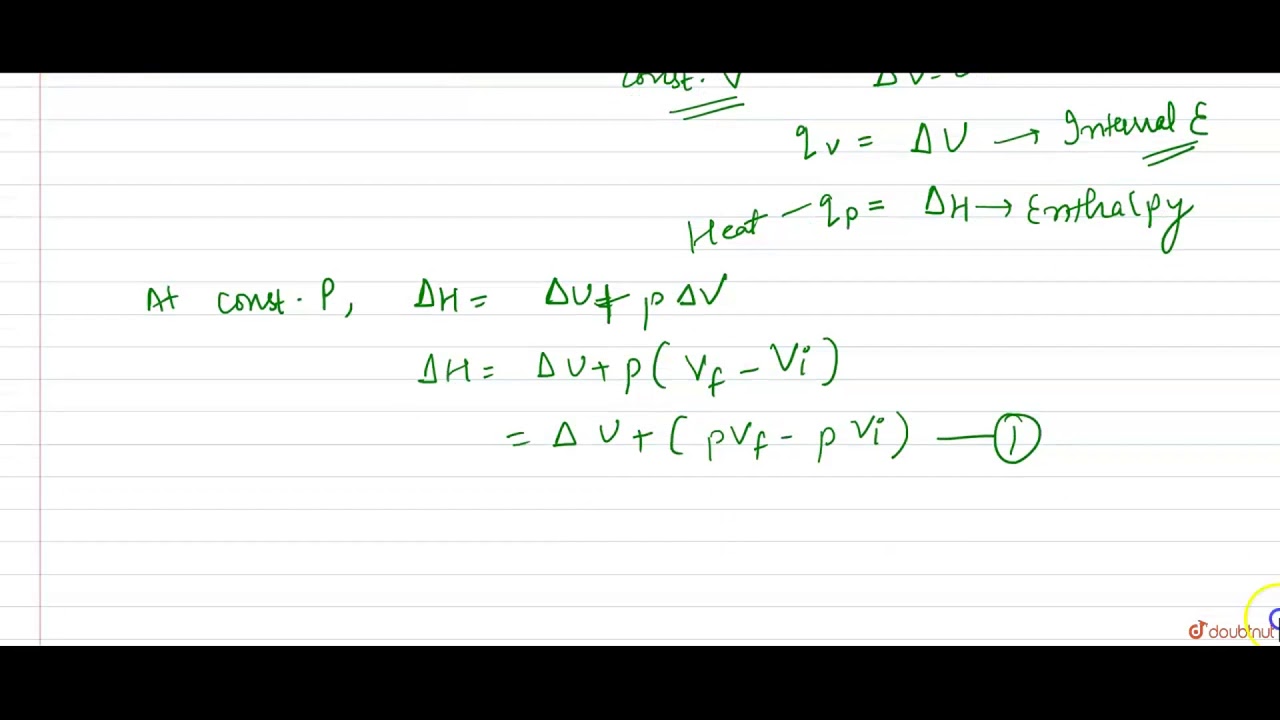
Derive the relationship between `Delta H \" and \" Delta U` for an ideal gas. Explain each term - YouTube
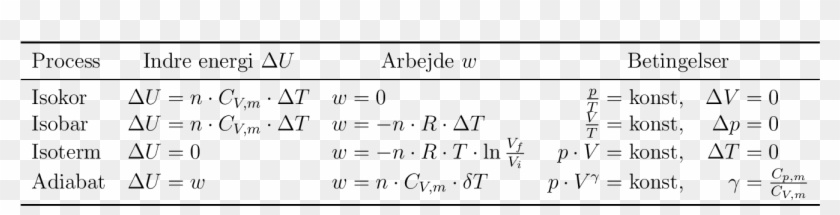
Compare the formula Cp - Cv = R for an ideal gas with the thermodynamics relation Delta U = Delta Q - P Delta V.

Thermodynamic Processes Illustrate how the 1 st law of thermodynamics is a statement of energy conservation Calculate heat, work, and the change in internal. - ppt download
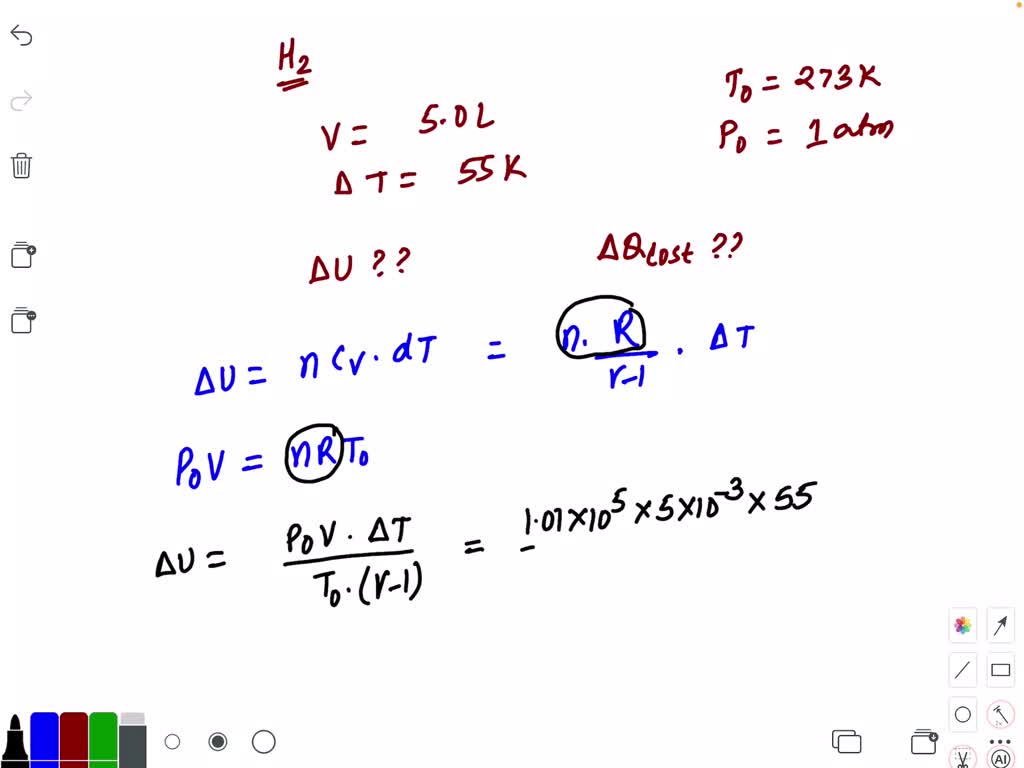
SOLVED:By the first law of thermodynamics, Q.=ΔU+A Here A=0, as the volume remains constant, So, Q=ΔU=(νR)/(γ-1) ΔT From gas law, p0 V=∨R T0 So, ΔU=(p0 V ΔT)/(T0(γ-1))=-0.25 kJ Hence amount of heat
55 calculate w,q and Delta u when 0.75 mole of an ideal gas expands isothermally and reversibly at27C from volume of 15litres to 25 litres

Relation between Delta h and Delta u for the reaction Pcl5 gives pcl3 + cl2 - Chemistry - Thermodynamics - 13237701 | Meritnation.com

What is the relationship between internal energy (delta U) and entropy ( delta S) - Chemistry - Thermodynamics - 13350243 | Meritnation.com
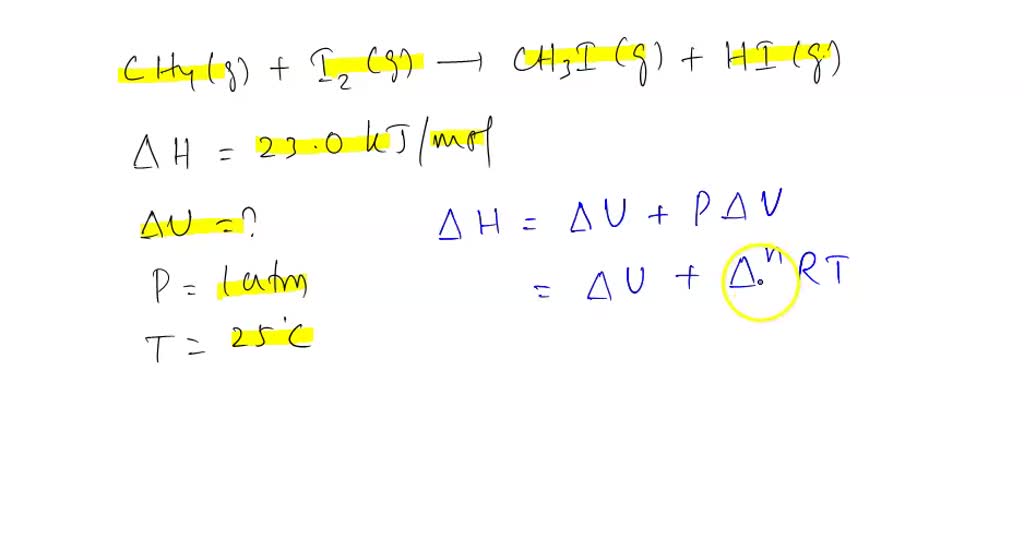
SOLVED: Calculate (delta)U for the following reaction at 1 atm and 25°C: CH4(g) + I2(g) —-> CH3I(g) + HI(g) (delta)H= +23.0 kJ/mol.

functional analysis - Why does $\int_{\Omega}\Delta u \frac{\partial u}{\partial t}dx=-\frac{1}{2}\frac{d }{d t}|u|^2_{H^1_0}$ hold? - Mathematics Stack Exchange